New battery systems (M/X battery) -
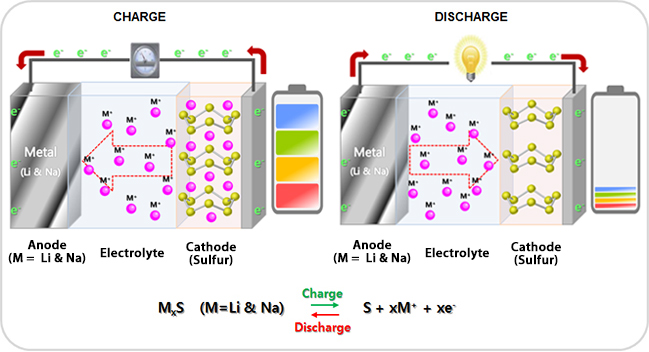
- Metal - sulfur battery (Metal=Li & Na)
-
Lithium-sulfur battery (Li-S battery) is a rechargeable battery having high energy density. By virtue of the low atomic weight of lithium and moderate weight of sulfur, Lithium-sulfur batteries are relatively light; about the density of water. Lithium-sulfur batteries may succeed lithium-ion cells because of their higher energy density and reduced cost from the use of sulfur. Currently the best Li-S batteries offer energy densities on the order of 500 Wh/kg, significantly better than most lithium-ion batteries which are in the 150 to 200 range. Li-S batteries with up to 1,500 charge and discharge cycles have been demonstrated, yet are not commercially available (as of early 2014). Sodium-sulfur battery (Na-S battery) is a type of molten-salt battery constructed from liquid sodium (Na) and sulfur (S). This type of battery has a high energy density, high efficiency of charge/discharge (89~92%) and long cycle life, and is fabricated from inexpensive materials. However, because of the operating temperatures of 300 to 350 °C and the highly corrosive nature of the sodium polysulfides, such cells are primarily suitable for large-scale non-mobile applications such as grid energy storage. -
 -
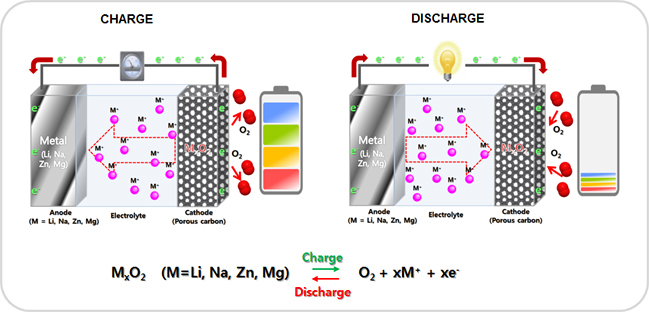
- Metal - air battery (Metal = Li, Na, Zn, Mg )
-
Lithium-air (Li-O2 battery) battery is a metal-air battery chemistry that uses the oxidation of lithium at the anode and reduction of oxygen at the cathode to induce a current flow. Originally proposed in the 1970s as a possible power source for electric vehicles, Li-air batteries recaptured scientific interest in the late 2000s due to advances in materials technology and an increasing demand for environmentally safe and oil-independent energy sources. The major appeal of the Li-air battery is the extremely high specific energy, a measure of the amount of energy a battery can store for a given weight. A lithium-air battery has an energy density (per kilo) comparable to gasoline per kilo. Li-air batteries gain this advantage in specific energy since they use oxygen from the air instead of storing an oxidizer internally. Lithium-air batteries have the potential of 5~15 times the specific energy of current lithium-ion batteries. Zinc-air batteries (non-rechargeable), and zinc-air fuel cells (mechanically-rechargeable) are metal-air batteries powered by oxidizing zinc with oxygen from the air. These batteries have high energy densities and are relatively inexpensive to produce. Zinc-air batteries have some properties of fuel cells as well as batteries: the zinc is the fuel, the reaction rate can be controlled by varying the air flow, and oxidized zinc/electrolyte paste can be replaced with fresh paste. Zinc-air batteries can be used to replace now discontinued 1.35 V mercury batteries (although with a significantly shorter operating life), which in the 1970s through 1980s were commonly used in photo cameras. Possible future applications of this battery include its deployment as an electric vehicle battery and as a utility-scale energy storage system. -
 |
|
|